In 1817, James Parkinson wrote his "Essay on the Shaking Palsy". He described a disorder manifest as "involuntary tremulous motion…with a propensity to bend the trunk forward and to pass from a walking to a running pace: the senses and intellects being uninjured." In 1877, Charcot codified PD as consisting of rigidity, a resting tremor described as "imitating the spinning of wool", and bradykinesia. In addition, he recognized that during the late stage of the disease the "mind would become clouded and the memory lost". Parkinson's disease is a relatively common neurodegenerative disease with an estimated prevalence of approximately 400 per 100,000 population (or 40 times the prevalence of ALS or HD). It usually becomes symptomatic during the sixth or seventh decade, and is progressively incapacitating with death occurring in about 10 years. Consistent with Charcot’s observation, dementia is over 5 times as frequent in elderly patients with PD as in age-matched controls, eventually occurring in approximately 40% of PD patients. PD is usually sporadic, and searches for environmental toxins have not been definitive (with the notable exception of MPTP). A family history is present in about 10-15% of cases, showing either autosomal dominant or recessive inheritance. The first gene linked to familial disease (PARK 1) codes for the α-synuclein protein, the second (PARK 2) for the “parkin” protein, and the third (PARK 5 – go figure) for the ubiquitin C-terminal hydrolase, L1 (UCH-L1). UCH-L1 hydrolyses bonds between ubiquitin molecules, providing ubiquitin monomers required to label abnormal proteins for proteosomal degradation.
Underdiagnosis is common; door-to-door surveys have detected up to 25% of cases in some studies. Overdiagnosis of Parkinson’s disease is similarly common; a recent autopsy study found the clinical diagnosis of PD to be correct in 76 out of 100 cases, with the other 24 cases consisting of other parkinsonian syndromes (mentioned below). This has practical importance, as non-PD causes of parkinsonism will not respond to standard PD pharmacotherapy (levodopa). It also would not be considered good form to execute PD-specific surgical interventions for patients suffering from other forms of parkinsonism.
Parkinsonism, that is the clinical syndrome associated with PD, is a result of loss of dopaminergic, catecholaminergic, and seritoninergic neurons of the brainstem, particularly those of the substantia nigra pars compacta and locus ceruleus. Clinical signs of Parkinson's disease become evident after loss of approximately 50% of nigral neurons (80% of striatal dopamine). There is often gross depigmentation of these regions due to neuronal degeneration. However, a large amount of residual pigment can be present within macrophages.
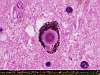
The characteristic (and diagnostically obligate) cytologic hallmark of PD is the Lewy body, which is an eosinophilic spherical inclusion, usually with a peripheral halo or concentric lamination, seen within the cytoplasm of surviving neurons in the brainstem pigmented nuclei as well as elsewhere within the nervous system.
Lewy bodies are composed primarily of a-synuclein, which has undergone a number of alterations, including phosphorylation, ubiquination, proteolysis, and cross-linking. The synucleins are a family of soluble proteins whose function is not (at present) well understood, but which came to attention when genetic mapping of a group of families with autosomal dominant PD revealed a mutation in the a-synuclein gene. Under conditions where the ubiquitin-proteosomal system is impaired, ubiquitinated proteins are transported along microtubules to perinuclear centrosomes. The centrosomes expand to become ‘aggresomes”, and are quarantined by a network of filaments. With continued impairment of proteolysis or overwhelming production of abnormal proteins, aggresomes continue to expand, forming Lewy bodies. Other parkinsonian syndromes (e.g. progressive supranuclear palsy,
corticobasal degeneration,
and multiple system atrophy) are not associated with Lewy body formation, though the latter shows a-synuclein inclusions within glial cells.
Degeneration of nigral neurons decreases the dopaminergic input to the corpus striatum, which increases the activity of striatal neurons projecting to the lateral globus pallidus. The disinhibited subthalamic excitatory activity results in increased activity of neurons in the medial globus pallidus and pars reticulata of the substantia nigra with inhibition of thalamocortical projection neurons resulting in akinesia, rigidity, and tremor. Understanding of this complex neuroanatomic system along with technological advances in neurosurgical machinery has led to development of deep brain stimulators as a treatment option for patients with medically intractable involuntary movements (dyskinesias) and severe fluctuations in motor coordination ("on/off" phenomenon). In a recent study, bilateral stimulation of the subthalamic nuclei resulted in a median improvement in motor score of 49 percent, while stimulation of the pars interna of the globus pallidus produced a median improvement of 37 percent. Other treatments currently under investigation include striatal transplantation of fetal tissue, and glutamate antagonists that act at the level of the corpus striatum.
Further reading
Lees AJ. The Parkinson chimera. Neurology. 2009 Feb 17;72(7 Suppl):S2-11.
Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009 Mar;8(3):270-9.
An Essay on the Shaking Palsy by James Parkinson
http://www.gutenberg.org/etext/23777