Among the resources in this section are 5 lectures providing an overview of the basic cells of the nervous system, and their reactions to damage.
For those who would prefer to read than listen:
The typical neuron is pyramidal or triangular-shaped with a large, round vesicular nucleus, prominent nucleolus, and basophilic material in the cytoplasm termed Nissl substance (Nissl substance corresponds to rough endoplasmic reticulum ultrastructurally). Each of these features are general indicators of active protein synthetic activity (e.g. neurotransmitters). The structurally different compartments of the neuron (axon, dendrites, cell body) subserve specific functions, with the overall role of transmitting information.
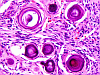
Eosinophilic neuronal degeneration (red neuron) refers to morphological alterations of neurons in the settting of acute neuronal (usually ischemic) necrosis. Changes consist of shrinkage of the cell body, nuclear pyknosis, disappearance of the nucleolus, loss of Nissl substance and intense cytoplasmic eosinophilia. The change is irreversible and appears about 12-24 hours following the ischemic insult.
Central chromatolysis (or “the axonal reaction”) - refers to a morphological change within the neuronal cell body that occurs with axotomy, in which the Nissl substance (the “chrome”) is displaced or disbursed (“lysed”) and the neuronal cell body swells with accumulation of cytoskeletal filaments and organelles. Distal to the axotomy, the axon degenerates – a process often referred to as Wallerian degeneration (though to purists true Wallerian degeneration is confined to the peripheral nervous system). Note that neuronal cell death is accompanied by degeneration of its axon; therefore, degeneration of the corticospinal tract following infarct in the motor cortex may be visualized grossly and may also be referred to as Wallerian degeneration.
Loss of neurons from whatever etiology may result in gross brain atrophy and is generally not very specific. Atrophy of selected neuronal populations, however, may suggest a specific disease (e.g. atrophy of the caudate nucleus in Huntington’s disease, frontotemporal atrophy in Pick’s disease). General cerebral atrophy also occurs with advanced age and is not necessarily pathological.
Neuronophagia is an acute process that follows fatal injury to neurons and is seen most prominently in toxic and infectious (mainly viral) lesions. At the light microscopic level, groups of activated microglial cells accumulate around dying neurons.
Intraneuronal inclusions occur in a variety of pathological processes, but particularly in neurodegenerative diseases and viral infections. Neurofibrillary tangles as seen in Alzheimer’s disease are composed of paired helical filaments containing abnormally phosphorylated tau protein. Lewy bodies are rounded eosinophilic inclusions that occur in substantia nigra neurons in Parkinson’s disease and contain alpha synuclein. Both of the pathologic inclusions appear to be the result of inadequate ubiquitin-mediated proteolysis of abnormally folded proteins. Recent evidence suggests that Lewy bodies are related to aggresomes, and may be cytoprotective. Negri bodies are eosinophilic cytoplasmic (viral) inclusions that occur in Rabies encephalitis.
Axonal swellings – If axons are primarily injured (with sparring of the neuronal cell body), impaired axonal transport will result in swelling of the axon. In local damage (e.g. transection, infarction, compression), the axons may swell quickly, and continue to do so in the early post-mortem interval. In diffuse axonal injury (DAI), which is classically encountered in motor vehicle accidents, swellings may be widespread and result in a persistent vegetative state. The mildest form of DAI is concussion, where recovery is the rule.
Astrocytes are glial cells with radiating cytoplasmic processes. In addition to their passive structural/supportive role, astrocytes induce the formation of the blood-brain barrier, regulate glutamate and potassium homeostasis, regulate synapse production by neurons, stimulate neurogenesis, and respond to injury by proliferating and increasing synthesis of glial fibrillary acidic protein (GFAP). GFAP is an intermediate filament expressed principally by astrocytes. Note that while astrocytes induce the formation of the blood brain barrier, the structural basis for the blood brain barrier are tight junctions of brain endothelial cells. Mature astrocytes are classically divided into fibrillary astrocytes (white matter astrocytes containing relatively coarse cytoplasmic processes) and protoplasmic astrocytes (gray matter astrocytes containing relatively fine cytoplasmic processes). The distinction between fibrillary and protoplasmic astrocytes appears to be largely morphological, as well-defined functional differences between the two are generally not described.
Synonymously referred to as reactive gliosis (which is perhaps a bit more accurate as microglial cells also participate), reactive astrocytosisis is the brain’s response to injury. It may be analogous to fibrosis outside the CNS. Instead of fibroblastic proliferation and extracellular collagen deposition that occurs in response to injury outside the CNS, the brain responds with astrocytic proliferation and intracellular accumulation of glial filaments. The result is a glial “scar” as opposed to a fibrous “scar.” Similar to cutaneous scars, areas of glial scarring feel firmer than surrounding brain or spinal cord parenchyma. Hence, areas of glial scarring were classically described as sclerotic, which explains such disease appelations as multiple sclerosis, hippocampal sclerosis, and amyotrophic lateral sclerosis. Recent data suggest that reactive astrocytes may also function to limit inflammation within the brain.
With elevated blood levels of ammonia, as occurs in hepatic failure, the astrocyte responds by decreasing GFAP synthesis and increasing production of glutamine synthetase, in an attempt to detoxify the extracellular microenvironment. The morphological manifestation is Alzheimer type 2 astrocytosis (completely unrelated to Alzheimer’s disease), where the astrocytes show enlarged vesicular, watery-looking nuclei and no visible cytoplasmic processes. Alzheimer type 2 astrocytosis is therefore a histological correlate of hepatic encephalopathy. Note: Alzheimer type 1 astrocytosis is usually described in the setting of Wilson’s disease (inherited hepatolenticular degeneration due to genetic abnormalities affecting copper metabolism and transport).
Corpora amylacea are proteoglycans that accumulate as a normal consequence of aging in astrocytic processes. By routine staining with hematoxylin and eosin, they are round and purple, with or without concentric rings. They may rarely be indicative of disease processes, either by accumulating in regions of chronic gliosis (e.g. hippocampal sclerosis in chronic epilepsy) or presenting in excessive size, abundance, or location in other unusual disorders (e.g. glycogen storage disease type IV, adult polyglucosan body disease).
Rosenthal fibers occur 1) adjacent to slowly expanding, often cystic masses; 2) in the walls of syringomyelic cavities; 3) in the astrocytic tumor cells of pilocytic astrocytoma; and 4) as the diagnostic hallmark of Alexander’s disease – a sporadic leukodystrophy of unknown etiology. Kindreds of familial Alexander’s disease containing a mutation in the gene for glial fibrillary acidic protein (GFAP) have been recently described, emphasizing the potential important role of GFAP in pathogenesis of Rosenthal fiber formation and Alexander disease. Rosenthal fibers contain GFAP and stress-related proteins such as Alpha-B crystallin, ubiquitin, and heat shock protein 27.
By routine staining, oligodendrocytes have small, round nuclei, while the cytoplasm cannot be discerned because it blends in with the background neuropil. The major role of oligodendrocytes is that of myelin production for CNS axons. Recall that oligodendrocytes can provide myelin for multiple internodal segments, while Schwann cells of the peripheral nervous system can provide myelin for only one internodal segment. Immune attack on oligodendrocytes forms the pathogenetic basis for multiple sclerosis.
Progressive multifocal leukoencephalopathy (PML) is caused by the JC virus. The JC virus is a papova virus and is in no way related to Creutzfeldt-Jakob disease or CJD. The JC virus accumulates in oligodendrocytes, resulting in oligodendroglial enlargement and oligodendroglial inclusion formation. Eventually, the infected oligodendrocytes lyse resulting in multiple foci of demyelination, hence the term progressive multifocal leukoencephalopathy.
Ependymal cells rest directly on brain parenchyma without an intervening basement membrane. They line the ventricular system and have a limited capacity, if any, to proliferate and respond to injury. Loss or destruction of ependymal cells leads to proliferation of subependymal astrocytes which results in subependymal nodules. If this process is widespread, as in patients with chronic hydrocephalus, the result is sometimes referred to as granular ependymitis, owing to the “granular” appearance it produces on the ventricular surface.
During development, the connective tissue of the primitive leptomeninges indents the ventricular lining at the roofs of the third and fourth ventricles and the medial walls of the cerebral hemispheres (lateral ventricles), inducing the formation of the choroid plexus. The ventricular lining becomes choroid plexus epithelium and the ingrowing connective tissue becomes the fibrovascular stroma of the choroid plexus. Mature choroid plexus epithelium consists of a single layer of cuboidal epithelial arranged in papillae with a fibrovascular core. The primary function of the choroid plexus is to produce cerebrospinal fluid. Overproduction of cerebrospinal fluid due by a papilloma of the choroid plexus is a rare cause of hydrocephalus.
Microglial cells are small, elongated bipolar cells that contain several cytoplasmic extensions emanating from each extremity. They compose up to 20% of the total glial cell population in the CNS. The cytoplasmic detail of microglial cells can only be demonstrated with special techniques. In spite of their name, microglial cells are derived from blood monocytes and form a network of antigen presenting cells in the CNS with a primary function in immune surveillance. They are sometimes referred to as “brain macrophages.”
With neuronal injury, microglial cells become activated, express class II MHC on their cell surface, and may transform into macrophages. Microglial nodules are localized clusters of activated microglial cells, and are most commonly seen in association with viral encephalitis. Neuronophagia, also common in viral encephalitis, represents a cluster of microglial cells that have transformed into macrophages and are in the process of phagocytosing a lethally injured neuron. The multinucleated microglial cell represents microglial cell activation and fusion in response to HIV infection. Cytokines and chemokines produced by infected microglial cells produces the neuronal dysfunction that characterizes HIV encephalitis. Microglial cells may play a similar effector role in producing mediating neuronal dysfuction and damage in Alzheimer’s disease and other neurodegenerative disorders.
Meningothelial cells are part of the arachnoid mater and serve to contain the subarachnoid space. Specialized collections of arachnoidal cells protrude into venous sinuses (arachnoid villi) and form a pressure dependent one-way valve that allows CSF to be reabsorbed into the systemic circulation. Inflammatory conditions, such as purulent meningitis, that obstruct the arachnoid villi may result in hydrocephalus.
In the normal adult, CSF is produced at a rate of 500 ml/day, while the total CSF volume is about 150 ml. Therefore, the CSF is renewed every 6 to 8 hours. The CSF is produced by the choroid plexus and is reabsorbed by the arachnoid villi. Hydrocephalus is defined as enlargement of the ventricles with an associated increase in the volume of CSF, and is most commonly due to obstruction of CSF flow rather than overproduction. Depending on the site of obstruction, hydrocephalus is classified into communicating and non-communicating types. Non-communicating hydrocephalus refers to obstruction within the ventricular system, usually at the cerebral aqueduct or foramen of Monroe, causing poor “communication” between the ventricles proximal and distal to the obstruction. In communicating hydrocepalus, the obstruction occurs somewhere in the subarachnoid flow, often at the arachnoid villi, such that the ventricular system is patent or “communicates.”
Further Reading
Jeans A, Esiri M. Brain histology. Pract Neurol. 2008 Oct;8(5):303-10.
Barres BA. The mystery and magic of glia: a perspective on their roles in
health and disease. Neuron. 2008 Nov 6;60(3):430-40.